Barrier Immunology
The scientific focus of the Barrier Immunology Group is to understand the cellular and molecular mechanisms regulating immune responses at our barrier surfaces, primarily the intestine. Broadly, we are interested in determining how different cell populations and niches within barrier tissues interact to support local immune homeostasis, the environmental and tissue derived factors that regulate barrier immune function and how alterations in this cross-talk contribute to the initiation and maintenance of barrier and systemic inflammation.

The intestinal mucosa represents the largest surface area of the body that is exposed to the outside environment and is continually exposed to foreign material derived from our diet and the trillions of microorganisms residing within the intestinal lumen.
The maintenance of intestinal homeostasis is dependent upon the immune system’s ability to remain tolerant to such material, while retaining the ability to mount appropriate immune responses to the many viral, parasitic, and bacterial pathogens that utilize the intestine as a primary site of entry into the host. A breakdown in such mechanisms is thought to contribute to the development and maintenance of inflammatory bowel disease (IBD; Crohn’s disease and ulcerative colitis).
While biologics such as TNF-a inhibitors have improved the treatment of IBD, many patients do not respond to such treatment, develop intolerable adverse events or lose the therapeutic effect over time. There is thus an urgent and unmet need for new directions and approaches to disease management and treatment.
Center for Intestinal Immune Regulation (CIIR)
Funding: NNF-Challenge grant
The overall aim of CIIR is to investigate the mechanisms by which intestinal fibroblasts regulate intestinal immune function in health and disease. CIIR is focused on testing two major hypotheses; (1) that intestinal fibroblasts play a key role in intestinal immune cell development, maintenance and function and that such processes are essential for maintaining intestinal immune homeostasis and (2) that alterations in intestinal fibroblast-immune cell crosstalk can contribute to the initiation and maintenance of IBD.
To test these hypotheses, CIIR brings together 4 collaborating groups (Agace (Copenhagen University), Prof. Flemming Bengtsen (Hvidovre Hospital), Ass. Prof. Lars Rønn Olsen (Technical University of Denmark) and Prof. Kathy McCoy (University of Calgary)) with expertise in gastroenterology, mucosal immunology and bioinformatics where we combine the analysis of human intestinal tissues using in-house developed isolation protocols in combination with single cell (transcriptional and proteomic) analysis and in vitro modelling with more mechanistic studies using state-of-the-art transgenic animal models (see Figure below).
The long-term goals of CIIR are to identify novel treatment modalities for IBD as well as biomarkers of disease severity and treatment response.
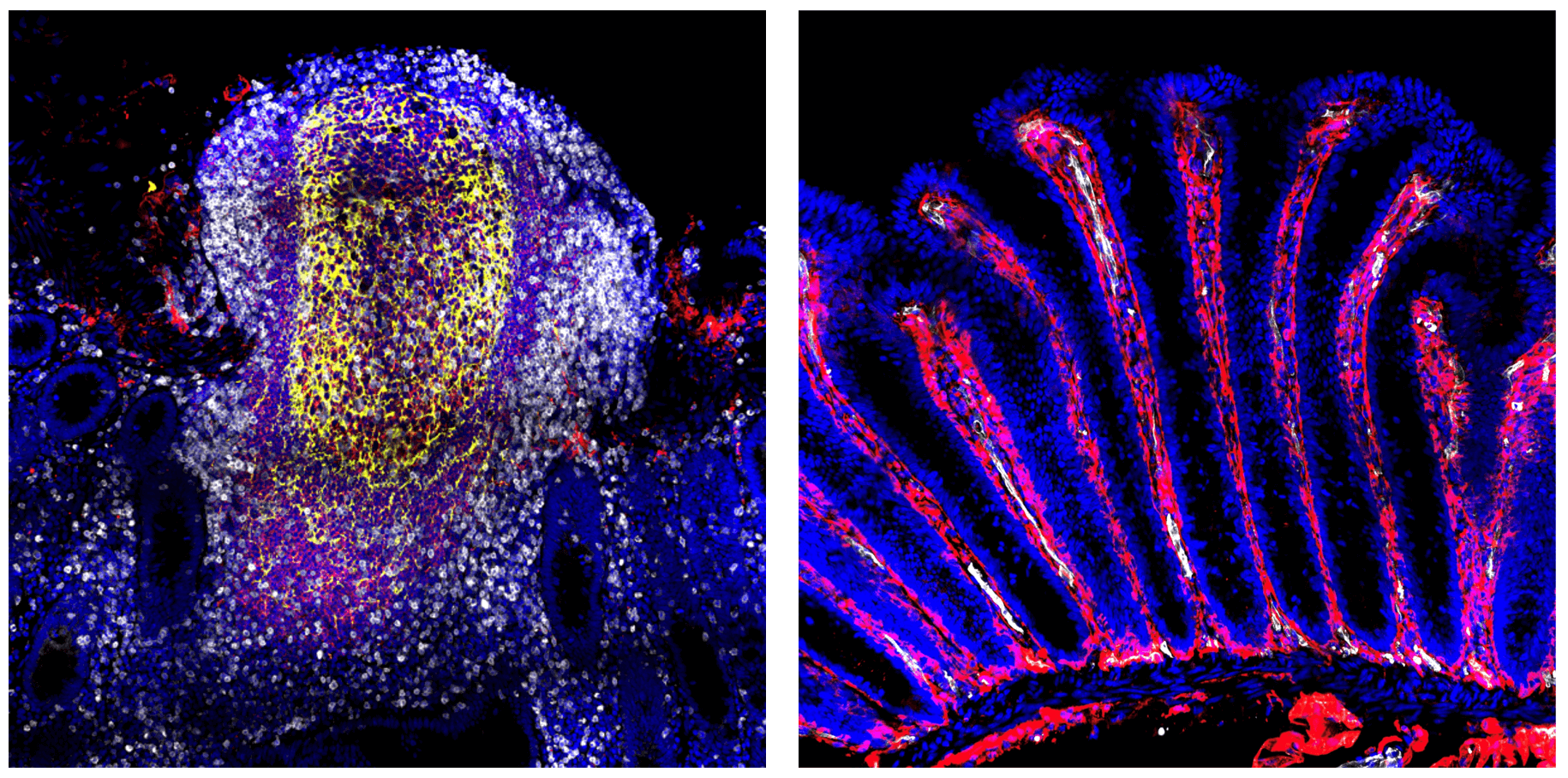
Figure: Immune compartments of the intestinal mucosa.
Immune niches within the intestinal mucosa include (1) the intestinal inductive sites termed the gut-associated lymphoid tissues (including the multi-follicular Peyer’s patches and isolated lymphoid follicles (left)) that represent the major sites of adaptive immune priming in the mucosa and (2) the intestinal effector sites, the epithelium and lamina propria (right) where adaptive and innate immune cells accumulate and carry out their effector functions. Immune cells within these environments reside within a dense network of structural cells including epithelial cells, fibroblasts, neuronal cells and blood vessels that together with immune cells help maintain intestinal function, immune homeostasis and intestinal barrier integrity.
An Atlas of intestinal immune niches in health and Crohn’s disease
Funding: The Leona M. and Harry B. Helmsley Charitable Trust, Gut Cell Atlas Consortium
As part of the Gut Cell Atlas (GCA) consortium and together with Prof. Eugene Butcher (Stanford University), we are generating a single cell transcriptional and proteomics atlas of immune niches of the human ileum and proximal colon in health and in Crohn’s disease, including human GALT (Gut associate lymphoid tissues; Peyer’s patches, mucosal and submucosal isolated lymphoid follicles), intestinal draining mesenteric lymph nodes and GALT-free intestinal lamina propria. Bioinformatic analysis of generated data sets as well as in vitro culturing techniques are focused on assessing antigen presenting cell diversity as well as adaptive immune cell priming and trafficking within intestinal inductive and effector sites in health and in Crohn’s disease.
Immune crosstalk along Gut-Skin axis
Funding: LEO Foundation Skin Immunology Research Center (SIC)
IBD is often associated with extra-intestinal co-morbidities. For example, approximately 1 in 10 patients with IBD display skin associated comorbidities including psoriasis, psoriatic arthritis, atopic dermatitis, pyoderma gangrenosum, and erythema nodosum. Despite this the underlying immunological processes linking intestinal and skin inflammation in these patients remains poorly understood. The aim of this project is, together with collaborators within SIC, to perform deep immunological profiling of affected skin and intestinal tissues from these patients to determine whether one can identify commonalities in inflammatory pathways and cell types across inflamed tissues.
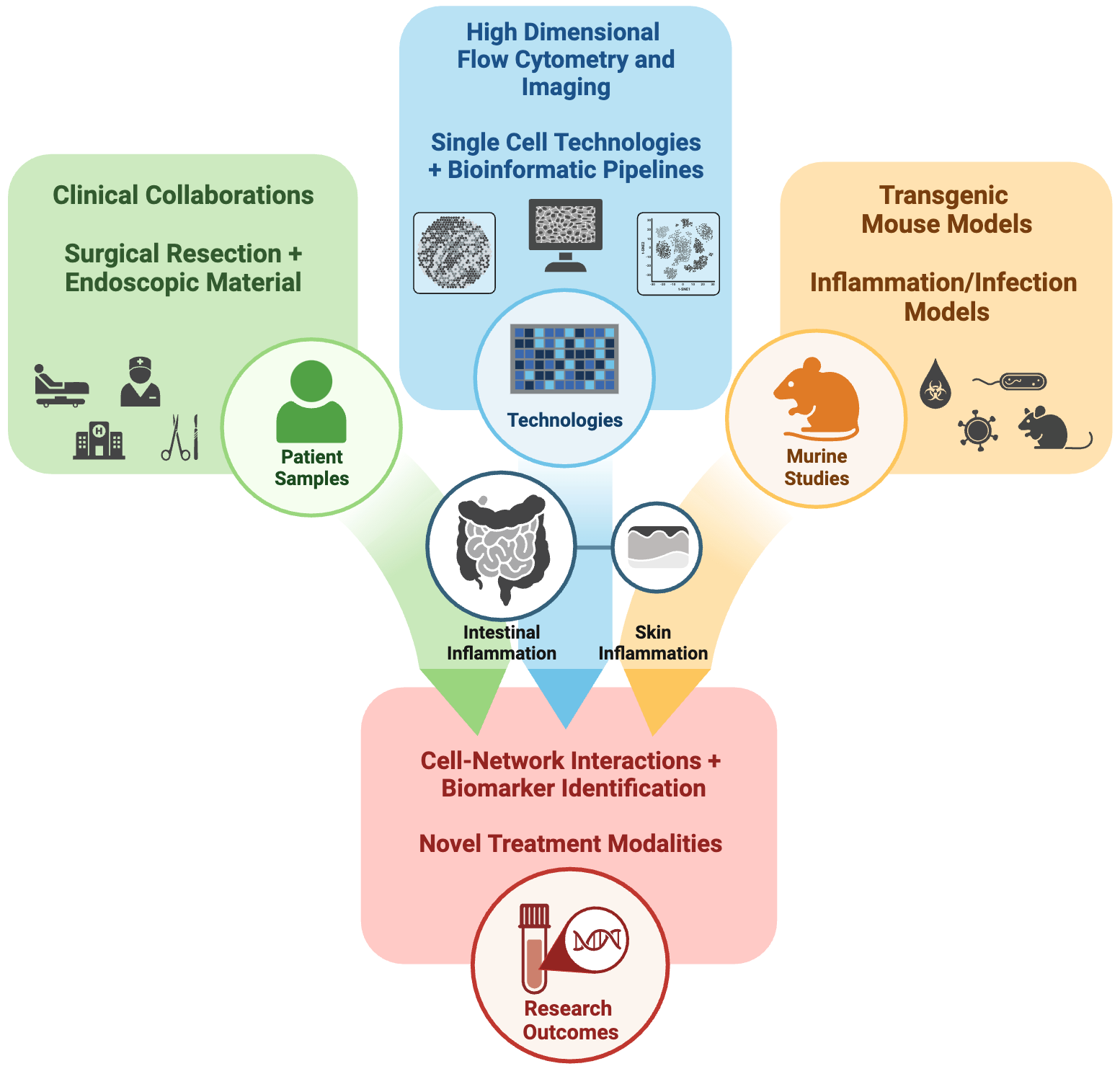 Figure: Overview of the Barrier Immunology Group
Figure: Overview of the Barrier Immunology Group
The Barrier Immunology Group integrates analysis of human clinical samples with mechanistic studies in in vitro and transgenic animal models to dissect immune processes at barrier surfaces. Technologies include the use of state-of the-art platforms in high-dimensional single cell proteomics (flow cytometry, CITE-seq) and transcriptomics with spatial imaging and dedicated bioinformatic pipelines.
SIC Announcement
Novo Nordisk Foundation Challenge Grant (2023 –2028). ‘Center for Intestinal Immune Regulation (CIIR)
Key Collaborators:
- Prof. Flemming Bengtsen - Hvidovre Hospital
- Ass. Prof. Lars Rønn Olsen - Technical University of Denmark
- Prof. Kathy McCoy - University of Calgary
Leona M. and Harry B. Helmsley Charitable Trust
Key Collaborators:
- Prof. Eugene Butcher – Stanford University
- Prof. Flemming Bengtsen – Hvidovre Hospital
- The Human Cell Atlas Consortium
Staff list
| Name | Title | Phone | |
|---|---|---|---|
| Andrew Stephen Brown | Postdoc | +4535321670 | |
| Christian Thomas Ashworth | PhD Fellow | ||
| Erik van Tilburg Bernardes | Postdoc Marie Curie | +4535322315 | |
| Faidra Papalamprou | PhD Fellow | ||
| Fredrik Viktor Junghus | Postdoc | ||
| Gina Ann Lindberg | PhD Fellow | +4535324368 | |
| Johanna Kabbert | Postdoc | +4535323340 | |
| Knut Kotarsky | Guest Researcher | ||
| Luiza Moraes Holst | Postdoc | ||
| Mads Damsgaard Wewer | External, Ph.d Student | ||
| Peter Tougaard | Postdoc | +4535337803 | |
| Theresa Mitterer | Research Assistant | +4535333688 | |
| Urs Michael Mörbe | Assistant Professor | +4535323446 | |
| Venla Anniina Väänänen | PhD Fellow |


